Synthesis and biological evaluation of cyclic derivatives of combretastatin A-4 containing group 14 elements†
Abstract
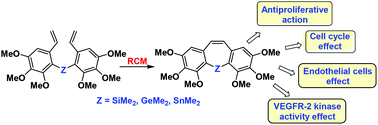
Several tricyclic compounds inspired by the structure of combretastatin A-4 and bearing group 14 elements have been synthesized by homocoupling lithiated aryl fragments followed by ring-closing metathesis. These tricyclic compounds and their diolefin precursors were evaluated for their antiproliferative action on the tumor cell lines HT-29, MCF-7, HeLa and A-549 and on the non-tumor cell line HEK-293. In addition, their effects on the cell cycle were also measured. The tricyclic compounds show antiproliferative activity similar to that of combretastatin A-4, even though they are not so active in arresting the cell cycle. However, some diolefin precursors are able to cause accumulation of cells in the G2/M phase in a higher percentage than combretastatin A-4 itself. Inhibition of endothelial tube formation and VEGFR-2 phosphorylation of some selected compounds is comparable to that of combretastatin A-4, particularly those of tin-containing compounds 23c and 26c, whose actions exceed those of sorafenib, a clinically used VEGFR-2 inhibitor.


 Please wait while we load your content...
Please wait while we load your content...