Stereocontrolled synthesis of polyhydroxylated bicyclic azetidines as a new class of iminosugars†
Abstract
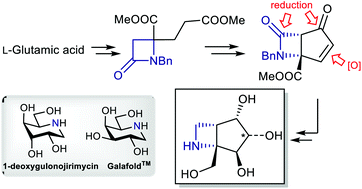
We report herein the development of a stereodivergent route towards polyhydroxylated bicyclic azetidine scaffolds, namely 6-azabicyclo[3.2.0]heptane derivatives. The strategy hinges on a common bicyclic β-lactam precursor, which is forged by way of a rare example of a cationic Dieckmann-type reaction, followed by IBX-mediated desaturation. Substrate-controlled diastereoselective oxidations then allow the divergent preparation of novel iminosugar mimics.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...