Synthesis and biochemical evaluation of two novel N-hydroxyalkylated cyclosporin A analogs†
Abstract
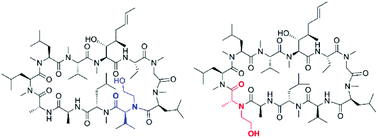
The cyclic undecapeptide cyclosporin A (CsA) is a widely used immunosuppressive agent. Its immunosuppressive properties arise from strong binding to cyclophilins (Cyp) followed by inhibition of the protein calcineurin (CaN) by the binary CsA/Cyp complex and subsequent negative regulation of T-cell activation. In the present study we show a novel way to modify the CsA ring by selective N-hydroxyalkylation of the residues Val5 and D-Ala8. Moreover, the influence of these structural CsA modifications on the ability of the CsA analogs to bind Cyp, to inhibit CaN and to penetrate membranes of living cells was investigated. Our results show that the Val5 N-substitution significantly improved compound cell-permeability and markedly diminished CaN inhibition by the binary CsA analog/CypA complex but to a lesser extent Cyp inhibition. In contrast, the N-alkylation of D-Ala8 gave a product with significantly reduced affinity for Cyp but its immunosuppressive effects remained similar to CsA. Possible explanations of the observed experimental data are provided by computational studies.


 Please wait while we load your content...
Please wait while we load your content...