Rapid and reversible hydrazone bioconjugation in cells without the use of extraneous catalysts†
Abstract
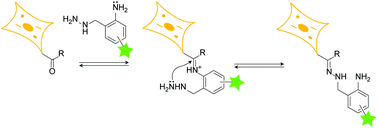
The amenability of hydrazone linkages to disassemble via either hydrolysis in mildly acidic aqueous solutions or transimination upon treatment with amine nucleophiles renders them extremely attractive for applications in chemical biology, drug delivery and materials science. Unfortunately, however, the use of hydrazones is hampered by the extremely slow intrinsic rates of their formation from their hydrazine and carbonyl precursors. Consequently, hydrazone formation is typically performed in the presence of a large excess of cytotoxic aniline-based nucleophilic catalysts, rendering hydrazones unsuitable for biological applications that entail their formation in cells. Herein, we report a hydrazine scaffold—o-amino benzyl hydrazine—that rapidly forms hydrazones via intramolecular nucleophilic catalysis, thereby obviating the use of extraneous catalysts. We demonstrate the use of this scaffold for rapid and reversible peptide and protein hydrazone bioconjugation and also for reversible fluorescent labeling of sialylated glycoproteins and choline lipids in mammalian cells.


 Please wait while we load your content...
Please wait while we load your content...