Recent advances in the annulation of Morita–Baylis–Hillman adducts
Abstract
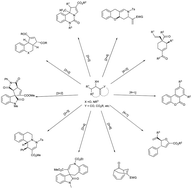
In this review, we summarize some of the most recent advances in the construction of cyclic compounds from the annulation of Morita–Baylis–Hillman (MBH) adducts, which have demonstrated their importance by possessing diverse functional groups. Significant examples including [3 + 2], [3 + 3], [3 + 4] and other cyclizations described herein with MBH adducts were proven to be efficient approaches for the preparation of diverse cyclic structure motifs. However, most of the reported strategies are based on the use of non-chiral catalysts/ligands, whilst stereoselective reactions remain largely unexplored. This area is still in its infancy and future research on MBH adducts will definitely benefit the organic chemistry community, especially for the synthesis of drug candidates and other molecules that might draw attention to materials sciences.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...