Silver(i)-catalyzed sequential hydroamination and Prins type cyclization for the synthesis of fused benzo-δ-sultams†
Abstract
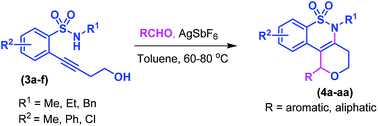
An intramolecular annulation strategy has been developed for the synthesis of tetrahydrobenzo[e]pyrano[4,3-c][1,2]thiazine derivatives by means of coupling of aldehydes with 2-(4-hydroxybut-1-yn-1-yl)-N-arylsulfonamides using a catalytic amount of silver hexafluoroantimonate in toluene at 80 °C. This is the first report on the synthesis of fused benzo-δ-sultam derivatives through C–N, C–O, and C–C bond formations. The reaction proceeds through a cascade of hydroamination and Prins type cyclization.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...