Chelation-assisted de-aryloxylative amination of 2-aryloxy quinolines: a new synthetic route to a key fragment of a bioactive PRMT5 inhibitor†
Abstract
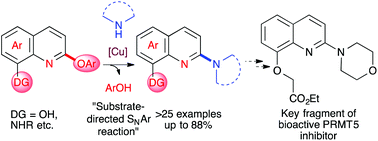
A highly regioselective de-aryloxylative amination of O- or N-chelating group-functionalized 2-aryloxy quinolines has been accomplished by means of a copper catalyst. The chelating functional groups of the substrate play a crucial role in directing the C-2-selective amination process, which proceeds through a novel aromatic nucleophilic substitution of the aryloxy group. The methodology provides expedient access to an important class of functionalized 2-aminoquinolines (up to 88% isolated yield) and was successfully applied for the synthesis of a key fragment of an important bioactive PRMT5 inhibitor.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...