Regio- and stereospecific Friedel–Crafts alkylation of indoles with spiro-epoxyoxindoles†
Abstract
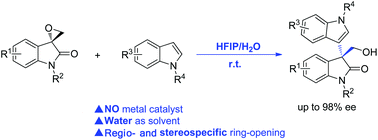
A highly efficient strategy for the regio- and stereospecific Friedel–Crafts alkylation of indoles with spiro-epoxyoxindoles has been developed in the mixed solvents of HFIP/H2O (1 : 9) without the use of catalysts. This protocol provides an atomically economical, catalyst-free and simple route for the construction of synthetically useful 3-(3-indolyl)-oxindole-3-methanols in high yields. Starting from optically active spiro-epoxyoxindoles a variety of enantiospecific 3-(3-indolyl)-oxindole-3-methanols could be obtained in high yields with complete retention of enantioselectivity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...