Highly enantioselective synthesis of α-tertiary chiral amino acid derivatives through rhodium-catalyzed asymmetric arylation of cyclic N-sulfonyl α-ketimino esters†
Abstract
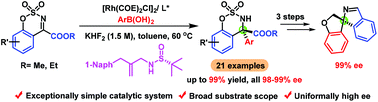
By employing an easily available, exceptionally simple sulfur-olefin ligand, a highly enantioselective rhodium-catalyzed arylation of cyclic N-sulfonyl α-ketimino esters with arylboronic acids has been developed. The reaction allows facile access to a wide range of α,α-gem-diaryl substituted chiral amino esters in excellent yields with extremely high enantioselectivities (uniformly 98–99% ee). The synthetic utility of this approach was highlighted by the efficient synthesis of a series of interesting molecules including unique chiral spirocycles bearing 2,3-dihydrobenzofuran and 1H-isoindole skeletons.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...