A “cross-stitched” peptide with improved helicity and proteolytic stability†
Abstract
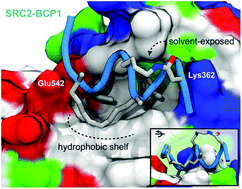
A new computational approach to obtain quantitative energy profiles for helix folding was used in the design of orthogonal hydrocarbon and lactam bicyclic peptides. The proteolytically stable, “cross-stitched” peptide SRC2-BCP1 shows nanomolar affinity for estrogen receptor α and X-ray crystallography confirms a helical binding pose.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...