A homodinuclear cobalt complex for the catalytic asymmetric Michael reaction of β-ketoesters to nitroolefins†
Abstract
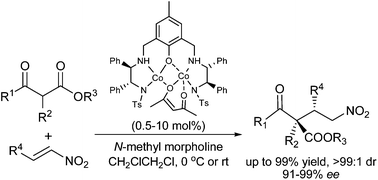
A homodinuclear Co2/aminophenol sulfonamide complex has been developed for the asymmetric Michael reaction of β-ketoesters with nitroolefins. This procedure is capable of tolerating a wide range of substrates and excellent results (up to 99% yield, >99 : 1 dr and 98% ee) can also be obtained. Moreover, the reaction could be carried out on a 50 mmol scale without any decrease in the enantioselectivity and reactivity. On the basis of the results of mechanistic studies, we proposed that the Co2/2a complex would be the active species and a possible catalytic cycle was described.


 Please wait while we load your content...
Please wait while we load your content...