Mixed carboxylic–sulfonic anhydride in reaction with imines: a straightforward route to water-soluble β-lactams via a Staudinger-type reaction†
Abstract
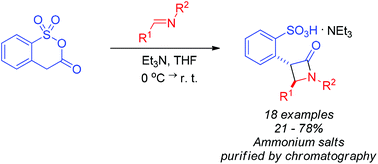
The first example of employing a mixed carboxylic–sulfonic anhydride in reaction with imines is reported. Unlike its well-studied isostere homophthalic anhydride, benzo[c][1,2]oxathiin-3(4H)-one 1,1-dioxide gave no product of a formal [4 + 2] cycloaddition and only followed an alternative reaction pathway toward β-lactams, presumably, via a formal [2 + 2] cycloaddition (a Staudinger-type reaction). Optimized reaction conditions involve the use of triethylamine as a base promoter, which also allows isolating the product β-lactam benzene sulfonic acids as respective triethylammonium salts by conventional column chromatography. The reaction shows some preference to trans-isomer formation; pure diastereomers can be isolated in some cases.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...