Tracking down protein–protein interactions via a FRET-system using site-specific thiol-labeling†
Abstract
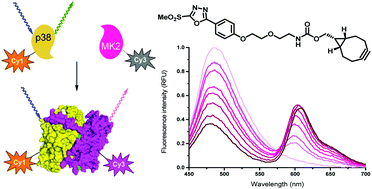
Förster resonance energy transfer is among the most popular tools to follow protein–protein interactions. Although limited to certain cases, site-specific fluorescent labeling of proteins via natural functions by means of chemical manipulations can redeem laborious protein engineering techniques. Herein we report on the synthesis of a heterobifunctional tag and its use in site-specific protein labeling studies aiming at exploring protein–protein interactions. The oxadiazole-methylsulfonyl functionality serves as a thiol specific warhead that enables easy and selective installation of fluorescent labels through a bioorthogonal motif. Mitogen activated protein kinase (MAPK14) and its substrate mitogen activated protein kinase activated kinase (MAPKAP2) or its docking motif, a 22 amino acid-long peptide fragment, were labeled with a donor and an acceptor, respectively. Evolution of strong FRET signals upon protein–protein interactions supported the specific communication between the partners. Using an efficient FRET pair allowed the estimation of dissociation constants for protein–protein and peptide–protein interactions (145 nM and 240 nM, respectively).


 Please wait while we load your content...
Please wait while we load your content...