The synthesis of non-racemic β-alkyl-β-aryl-disubstituted allyl alcohols and their transformation into allylamines and amino acids bearing a quaternary stereocenter†
Abstract
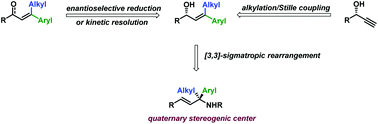
A synthesis of non-racemic β-alkyl-β-aryl allyl alcohols and their transformation into allylamines bearing a quaternary stereogenic center is reported. The allyl alcohols were prepared either by Cu-catalyzed enantioselective reduction of enones or by sequential alkylation/hydrostannylation/Stille coupling of non-racemic propargyl alcohols. The prepared β-alkyl-β-aryl allyl alcohols were converted (after carbamoylation) to the corresponding allylamine derivatives through cyanate-to-isocyanate rearrangement/nucleophilic addition with complete chirality transfer. Varying the nucleophilic agents allowed the preparation of various allylamine derivatives, including carbamates, amides, formamides, ureas, and free amines. The ozonolysis/oxidation of the resulting allylamines provided non-racemic quaternary α-amino acids.


 Please wait while we load your content...
Please wait while we load your content...