Promoter free allylation of trichloroacetimidates with allyltributylstannanes under thermal conditions to access the common 1,1′-diarylbutyl pharmacophore†
Abstract
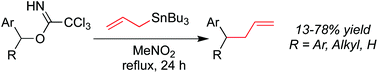
1,1′-Diarylbutyl groups are a common pharmacophore found in many biologically active small molecules. To access these systems under mild conditions, the reaction of diarylmethyl trichloroacetimidates with allyltributylstannanes was explored. Simply heating allyltributylstannane with the trichloroacetimidate resulted in substitution of the imidate with an allyl group. Unlike other methods used to access these systems, no strong base, transition metal catalyst, Brønsted acid or Lewis acid promoter was required to affect the transformation. Conversions are best with electron rich benzylic trichloroacetimidate systems, where excellent yields are achieved just by refluxing the reactants together in nitromethane.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...