Synthesis of CF3-containing spiro-epoxyoxindoles via the Corey–Chaykovsky reaction of N-alkyl isatins with Ph2S+CH2CF3OTf−†
Abstract
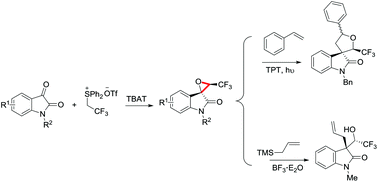
CF3-containing spiro-epoxyoxindoles were successfully prepared via the Corey–Chaykovsky reaction of N-alkyl isatins with the ylide generated from Ph2S+CH2CF3OTf− with almost exclusive diastereoselectivity. Further derivatizations of these spiro-epoxyoxindoles were explored via a photochemical reaction or Lewis acid-promoted allylation.


 Please wait while we load your content...
Please wait while we load your content...