Facile Cu(ii)-mediated conjugation of thioesters and thioacids to peptides and proteins under mild conditions†
Abstract
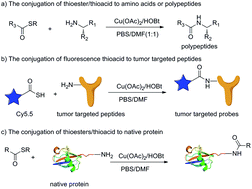
The bioconjugation of peptide derivatives such as polypeptides, peptide-based probes and proteins is a vibrant area in many scientific fields. However, reports on metal-mediated chemical methods towards native peptides especially non-engineering protein modification under mild conditions are still limited. Herein, we describe a novel Cu(II)-mediated strategy for the conjugation of thioesters/thioacids to peptides under mild conditions with high functional group tolerance. Based on this strategy, polypeptides, even peptide-based fluorescent probes, can be efficiently constructed. Finally, the selective modification of lysine residues of native Ub with thioesters could be realized and complete conjugation of Ub could be achieved even under equivalent Cu(II). These promising results could greatly expand Cu(II)-mediated reaction strategies on chemical biology and molecular imaging.


 Please wait while we load your content...
Please wait while we load your content...