Stereodivergent synthesis of 5-aminopipecolic acids and application in the preparation of a cyclic RGD peptidomimetic as a nanomolar αVβ3 integrin ligand†
Abstract
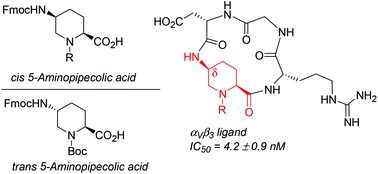
A stereodivergent strategy was devised to obtain enantiopure cis and trans 5-aminopipecolic acids (5-APAs) in suitably protected forms to be employed in peptide synthesis as conformationally constrained α- and δ-amino acids. The cis isomer was used as a δ-amino acid to construct a cyclic RGD-containing peptidomimetic, the ability of which to compete with biotinylated vitronectin for binding with the isolated αVβ3 integrin was measured (IC50 = 4.2 ± 0.9 nM). A complete 1H NMR and computational conformational analysis was performed to elucidate the reasons for the high affinity of this cyclic peptidomimetic in comparison with cilengitide.


 Please wait while we load your content...
Please wait while we load your content...