Metal-free photocatalyzed aerobic oxidative Csp3–H functionalization of glycine derivatives: one-step generation of quinoline-fused lactones†
Abstract
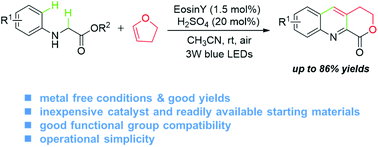
A metal-free aerobic oxidative dehydrogenative coupling/annulation tandem reaction of glycine derivatives to 2,3-dihydrofuran is disclosed. This process involves the synergistic performance of organic-dye-mediated photoredox catalysis and acid catalysis. This reaction provides rapid and facile access to a series of valuable quinoline-fused lactones at room temperature under an air atmosphere. Moreover, the reaction could be performed on a 20 mmol scale, without obvious reduction of the yield.


 Please wait while we load your content...
Please wait while we load your content...