2-Deoxyglycosyl 3-benzoylpropionates as novel donors for the direct and stereoselective synthesis of 2-deoxy-glycosides†
Abstract
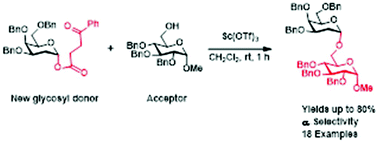
Lewis acid mediated stereoselective synthesis of 2-deoxy-O-glycosides has been demonstrated using 2-deoxyglycosyl 3-benzoylpropionates as novel glycosyl donors. These newly developed donors are easily synthesized from simple glycals, are stable at room temperature and react with ease to provide products with high stereoselectivity. These donors can be successfully utilized with all types of acceptors (primary, secondary and tertiary alcohols) for the synthesis of 2-deoxy-glycosides. Additionally, these newly developed glycosyl donors are also efficient for the synthesis of trisaccharides.

 Please wait while we load your content...
Please wait while we load your content...