Synthesis of fused pyrroles containing 4-hydroxycoumarins by regioselective metal-free multicomponent reactions†
Abstract
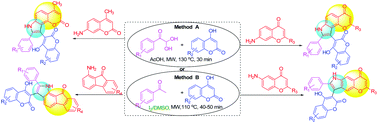
The reaction of arylglyoxals, 4-hydroxycoumarin, and aromatic amines such as 7-amino-2-methylchromone, 6/7-aminoflavone, 7-amino-4-methylcoumarin, 1-amino-9-fluorenone, 1-aminoanthraquinone and aniline derivatives in acetic acid medium under microwave conditions provides the corresponding regioselective fused pyrroles having hydroxycoumarin and aryl substituents. Alternatively, we have developed another method using in situ arylglyoxals from acetophenone derivatives by I2/DMSO promoted C–H oxidation followed by one-pot three component cyclization reactions to provide similar fused pyrroles. Using both the methods a series of novel pyrroles fused with pharmacologically important chromone, flavone, coumarin, fluorenone, and anthraquinone moieties were synthesized under metal-free reaction conditions in good to very good yields within a short reaction time. The structures of the synthesized fused pyrroles have been unambiguously confirmed by spectroscopic techniques, mass analysis and single crystal XRD.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...