One-pot synthesis of 3-fluoroflavones via 1-(2-hydroxyphenyl)-3-phenylpropane-1,3-diones and selectfluor at room temperature†
Abstract
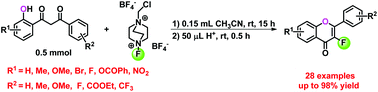
A concise and efficient one-pot strategy to synthesize 3-fluoroflavones was developed. 1-(2-Hydroxyphenyl)-3-phenylpropane-1,3-diones were fluorinated using selectfluor with a small amount of CH3CN at room temperature, followed by cyclization and dehydration in the presence of a trace amount of conc. H2SO4 to provide 3-fluoroflavones. The desired 3-fluoroflavone analogues were obtained in moderate to excellent yields. This strategy tolerated a wide range of functional groups and did not need sophisticated instruments or tedious substrate preparation.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...