Michael addition of carbonyl compounds to nitroolefins under the catalysis of new pyrrolidine-based bifunctional organocatalysts†
Abstract
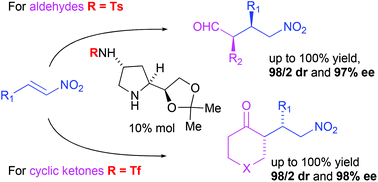
Novel bifunctional pyrrolidine-based organocatalysts for the asymmetric Michael addition of carbonyl compounds to nitroolefins have been synthesised from homoallylamines, which are easily obtained from (R)-glyceraldehyde as a chiral precursor. Under optimal reaction conditions, these bifunctional organocatalysts showed a high catalytic efficiency (almost quantitative yield in most cases) and stereoselectivity in the Michael addition reactions of a variety of aldehydes (up to 98 : 2 dr and 97% ee) and ketones (up to 98 : 2 dr and 99% ee) to nitroolefins.


 Please wait while we load your content...
Please wait while we load your content...