Peroxy mediated Csp2–Csp3 dehydrogenative coupling: regioselective functionalization of coumarins and coumarin-3-carboxylic acids†
Abstract
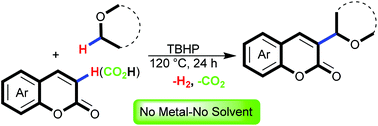
A regioselective direct alkylation of coumarins at C-3 via cross-dehydrogenative coupling of unactivated Csp2–Csp3 bonds is developed. This protocol employs tert-butyl hydroperoxide as the sole reagent of the reaction to combine coumarins and ethers in reasonable yields under metal- and solvent-free reaction conditions. This protocol also worked well with coumarin-3-carboxylic acids to unveil a rare instance of a catalyst-free tandem alkylation/decarboxylation reaction with conservation of the double bond.


 Please wait while we load your content...
Please wait while we load your content...