A three-dimensional nickel–chromium layered double hydroxide micro/nanosheet array as an efficient and stable bifunctional electrocatalyst for overall water splitting†
Abstract
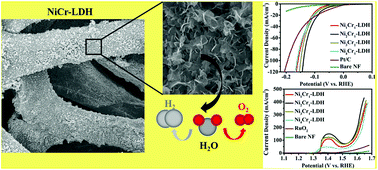
The development of bifunctional and stable non-noble metal electrocatalysts for the high-performance hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) is very important and challenging for renewable energy. Herein, for the first time, a nickel–chromium layered double hydroxide (NiCr-LDH) nanosheet array was developed as a bifunctional electrocatalyst towards overall water splitting. By tuning different Ni/Cr ratios of LDHs, the optimized Ni2Cr1-LDH shows extraordinary HER activity with an ultralow overpotential of 138 mV at 100 mA cm−2, compared with all of the reported Ni-based LDHs (NiFe-LDH, NiCo-LDH, NiMn-LDH, NiTi-LDH, NiV-LDH etc.) and even outperforming Pt/C catalysts. The small overpotential of 319 mV at 100 mA cm−2 for the OER and outstanding durability at 1.55 V (vs. RHE) for 30 hours can also be achieved for Ni2Cr1-LDH. Notably, a two-electrode electrolyzer with a Ni2Cr1-LDH bifunctional electrocatalyst as both the anode and the cathode can work for at least 30 hours with a cell voltage of merely 1.55 V at 10 mA cm−2. Both experimental and density functional theoretical calculations show that the Cr3+ ions within the LDH layer serve as charge transfer sites and thus effectively boost the intrinsic electrochemical activity. Therefore, this work provides a new NiCr-LDH system as a more efficient metal hydroxide for bifunctional water splitting electrocatalyst.


 Please wait while we load your content...
Please wait while we load your content...