Where do photogenerated holes at the g-C3N4/water interface go for water splitting: H2O or OH−?†
Abstract
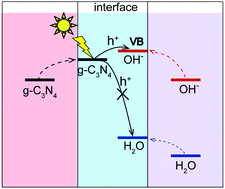
Graphitic carbon nitride (g-C3N4), a metal-free two-dimensional photocatalyst, has drawn increasing attention due to its application in photocatalytic water splitting. However, its quantum efficiency is limited by the poor performance of the oxygen evolution reaction (OER). Therefore, it is important to clarify the behavior of photogenerated holes in the OER. In this work, we investigate the energy level alignment using the GW method and the exciton properties using the Bethe–Salpeter equation within the ab initio many-body Green's function theory at the g-C3N4/water interface. We found that the g-C3N4 substrate can elevate energy levels of OH− and H2O molecules at the interface by up to 0.6 eV. This effect can make the electronic levels of OH− surpass the valence band maximum (VBM) of g-C3N4. However, orbital energies of H2O molecules remain far below the VBM of g-C3N4. This indicates that a photogenerated hole after exciting g-C3N4 can relax to OH− instead of neutral H2O. Moreover, OH− could be directly oxidized through electron transfer from OH− to g-C3N4 by light near the optical absorption edge of g-C3N4, which is beneficial for efficient carrier separation at the interface.


 Please wait while we load your content...
Please wait while we load your content...