Electrocatalysis of polysulfide conversion by conductive RuO2 nano dots for lithium–sulfur batteries†
Abstract
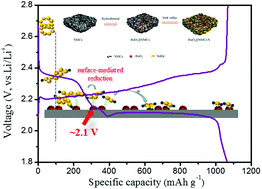
The practical use of the rechargeable lithium–sulfur (Li–S) battery is still restricted by poor cycle life and rate performance caused by the shuttle of soluble redox intermediates and low conductivity of S/Li2S. A comprehensive approach is to tune the multi-electron redox reactions and construct reversible chemical bonds with polysulfide intermediates. In this study, RuO2 nano dots (NDs) are proposed to anchor polysulfides, trigger the surface-mediated reduction of polysulfides and to facilitate the formation of Li2S2/Li2S through its catalytic effect for the first time. When serving as the sulfur host, the RuO2 NDs can retard the shuttle of polysulfides, accelerate the redox reaction of polysulfides, and therefore result in improved sulfur utilization and enhanced rate performance. The designed RuO2@NMCs/S ternary electrodes with high sulfur loading of 70 wt% could achieve a low decay rate of 0.07% per cycle for 500 cycles at a 0.5 C-rate. Realized by fast electrode kinetics, the reversible capacity of 634 mA h g−1 is attained at a high C-rate of 5 C. Overall, this strategy sheds new light on the oxide mediators for reversible modulation of electrochemical reactions in lithium–sulfur (Li–S) batteries.


 Please wait while we load your content...
Please wait while we load your content...