Preparation of gas phase naked silver cluster cations outside a mass spectrometer from ligand protected clusters in solution†
Abstract
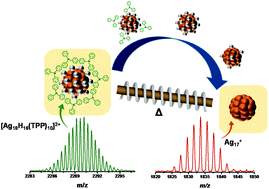
Gas phase clusters of noble metals prepared by laser desorption from the bulk have been investigated extensively in a vacuum using mass spectrometry. However, such clusters have not been known to exist under ambient conditions to date. In our previous work, we have shown that in-source fragmentation of ligands can be achieved starting from hydride and phosphine co-protected silver clusters leading to naked silver clusters inside a mass spectrometer. In a recent series of experiments, we have found that systematic desorption of ligands of the monolayer protected atomically precise silver cluster can also occur in the atmospheric gas phase. Here, we present the results, wherein the [Ag18H16(TPP)10]2+ (TPP = triphenylphosphine) cluster results in the formation of the naked cluster, Ag17+ along with Ag18H+ without mass selection, outside the mass spectrometer, in air. These cationic naked metal clusters are prepared by passing electrosprayed ligand protected clusters through a heated tube, in the gas phase. Reactions with oxygen suggest Ag17+ to be more reactive than Ag18H+, in agreement with their electronic structures. The more common thiolate protected clusters produce fragments of metal thiolates under identical processing conditions and no naked clusters were observed.


 Please wait while we load your content...
Please wait while we load your content...
