Exploiting poly(α-hydroxy acids) for the acid-mediated release of doxorubicin and reversible inside–out nanoparticle self-assembly†
Abstract
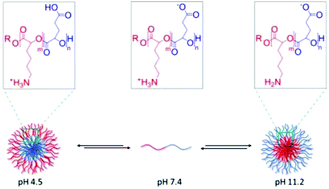
Biodegradable poly(α-hydroxy acid) copolyesters consisting of benzyl-protected glutamic acid and carboxybenzyl-protected lysine derived blocks possess the capability to self-assemble to form stable nanoparticles in aqueous solution (pH 7.4), that are able to withhold doxorubicin, prior to its directed release in acidic solution. Such pH-responsive nanoparticles are non-toxic against a panel of human breast cancer cell lines, but demonstrated comparable toxicities to free doxorubicin when loaded with doxorubicin. Significantly, comparable efficacy to free doxorubicin was observed even against triple negative breast cancer cells, highlighting the potential of the materials generated as drug delivery vehicles for cancer treatment. Facile block copolymer deprotection resulted in a polymer that presents an altered self-assembly/disassembly profile; forming nanoparticles when stored in either acidic or alkaline solution, but undergoing self-disassembly when added to aqueous solution of pH 7.4. This second polymer highlights the considerable versatility that poly(α-hydroxy acids) inherently possess.


 Please wait while we load your content...
Please wait while we load your content...