Controllable dynamics of oxygen vacancies through extrinsic doping for superior catalytic activities†
Abstract
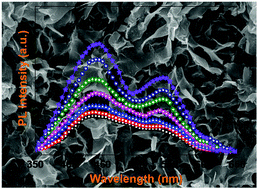
Due to its strong redox ability, high stability, cost effectiveness and non-toxicity, cerium oxide (CeO2) has been extensively researched as an active photocatalyst material. The underlying photocatalytic reactions are mostly associated with the transportation of oxygen ions through vacancies, but the actual transport phenomenon had not been clearly understood. In this work, gadolinium (Gd) is sequentially doped into CeO2 to investigate how extrinsic doping can modulate oxygen vacancies in CeO2 and influence photocatalytic activities. From our investigations, it was found that the Gd doping may induce structural symmetry breaking leading to a pure CeO2 fluorite structure that transforms mobile oxygen vacancies into clustered or immobile vacancies. When the vacancies were set as “mobile” (for Gd doping levels ≤15 at%), maximum photocatalytic activities were obtained. In contrast, suppressed photocatalytic efficiencies were noted for higher Gd doping levels (20 at% or more). The results reported in this research may provide an extra degree of freedom in the form of extrinsic doping to configure the oxygen vacancy defects and their mobility to achieve better catalytic efficiencies.


 Please wait while we load your content...
Please wait while we load your content...