Evidence of extended cation solubility in atomic layer deposited nanocrystalline BaTiO3 thin films and its strong impact on the electrical properties†
Abstract
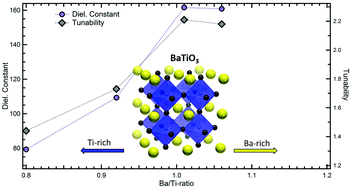
Thin films of ≈50 nm thickness with Ba/Ti-ratios ranging from 0.8 to 1.06 were prepared by depositing alternating layers of Ba(OH)2 and TiO2. Annealing at 750 °C promoted the solid–solid transformation into polycrystalline BaTiO3 films containing a mixture of the perovskite and the hexagonal polymorphs with average crystallite sizes smaller than 14 nm and without impurity phases. This, together with an increase of the cubic lattice parameters for Ba-rich films, suggests an extended metastable solubility range for the perovskite-phase in these nanocrystalline thin films on both sides of the stoichiometric composition. Mapping of the cation distribution utilizing energy-filtered transmission electron microscopy corroborates defect accommodation within the BaTiO3 grains. While the cation off-stoichiometry in thermodynamic equilibrium is negligible for BaTiO3, the metastable extended solubility range in the thin films can be directly correlated to the low annealing temperature and nanocrystalline nature. The leakage current behavior can be explained by the formation of Schottky defects for nonstoichiometric films, and the cation ratio has a distinct impact on the dielectric properties: while excess-BaO has a marginal detrimental effect on the permittivity, the dielectric constant declines rapidly by more than 50% towards the Ti-rich side. The present findings highlight the importance of compositional control for the synthesis of nanocrystalline BaTiO3 thin films, in particular for low annealing and/or deposition temperatures. Our synthesis approach using alternating layers of Ba(OH)2 and TiO2 provides a route to precisely control the cation stoichiometry.


 Please wait while we load your content...
Please wait while we load your content...