Complex strain induced structural changes observed in fibrin assembled in human plasma
Abstract
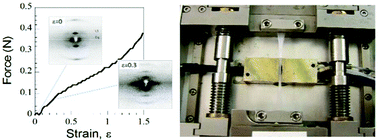
The structure of the core scaffold of blood clots, the interlinked 3-dimensional network of fibrin fibers, is modified by mechanical forces generated by platelet driven clot retraction, wound repair and shear stress through blood flow. Here X-ray diffraction is used to investigate how uniaxial strain, ε (ε = extension/original length), alters fiber structure in highly aligned human plasma clots covalently cross-linked by Factor XIIIa. Three stretch sensitive axially repeating structures are identified. Firstly, the foundation structure with an initial ≈22 nm axial repeat stretches, fades then disappears at ε ≈ 0.40. A second, lengthened transitory structure emerges at the low strains (ε ≈ 0.20) believed to be developed by cells. Finally, a third shortened structure appears after relaxation. Simultaneously as strain progresses an increasing fraction of molecules become axially disordered. Weak off-axis diffraction maxima indicate the presence of lateral ordering up to ε = 0.40 that partially recovers after relaxation. The reappearance of both axial and lateral order on relaxation demonstrates a surprising resilience in structure. In view of the range and importance of fibrin's functions, this structural heterogeneity, triggered in vivo by cell traction or shear stress, is likely to be of clinical significance.


 Please wait while we load your content...
Please wait while we load your content...