Control of the microstructure and surface chemistry of graphene aerogels via pH and time manipulation by a hydrothermal method†
Abstract
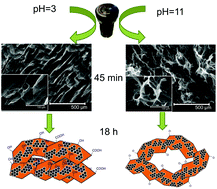
Three-dimensional graphene aerogels of controlled pore size have emerged as an important platform for several applications such as energy storage or oil–water separation. The aerogels of reduced graphene oxide are mouldable and light weight, with a porosity up to 99.9%, consisting mainly of macropores. Graphene aerogel preparation by self-assembly in the liquid phase is a promising strategy due to its tunability and sustainability. For graphene aerogels prepared by a hydrothermal method, it is known that the pH value has an impact on their properties but it is unclear how pH affects the auto-assembly process leading to the final properties. We have monitored the time evolution of the chemical and morphological properties of aerogels as a function of the initial pH value. In the hydrothermal treatment process, the hydrogel is precipitated earlier and with lower oxygen content for basic pH values (∼13 wt% O) than for acidic pH values (∼20 wt% O). Moreover, ∼7 wt% of nitrogen is incorporated on the graphene nanosheets at basic pH generated by NH3 addition. To our knowledge, there is no precedent showing that the pH value affects the microstructure of graphene nanosheets, which become more twisted and bent for the more intensive deoxygenation occurring at basic pH. The bent nanosheets attained at pH = 11 reduce the stacking by the basal planes and they connect via the borders, hence leading eventually to higher pore volumes. In contrast, the flatter graphene nanosheets attained under acidic pH entail more stacking and higher oxygen content after a long hydrothermal treatment. The gravimetric absorption capacity of non-polar solvents scales directly with the pore volume. The aerogels have proved to be highly selective, recyclable and robust for the absorption of nonpolar solvents in water. The control of the porous structure and surface chemistry by manipulation of pH and time will also pave the way for other applications such as supercapacitors or batteries.


 Please wait while we load your content...
Please wait while we load your content...