Passive membrane penetration by ZnO nanoparticles is driven by the interplay of electrostatic and phase boundary conditions†
Abstract
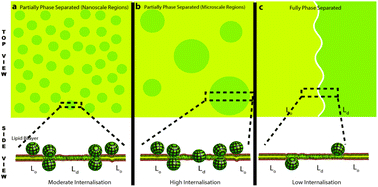
The internalization of nanoparticles through the biological membrane is of immense importance for biomedical applications. A fundamental understanding of the lipid specificity and the role of the membrane biochemical and physical forces at play in modulating penetration are lacking. The current understanding of nanoparticle–membrane interaction is drawn mostly from computational studies and lacks sufficient experimental evidence. Herein, using confocal fluorescence imaging and potentiometric dye-based fluorimetry, we first investigated the interaction of ZnONP in both multi-component and individual lipid membranes using cell-like giant unilamellar vesicles to dissect the lipid specificity; also, we investigated the changes in membrane order, anisotropy and hydrophobicity. ZnONP was found to interact with phosphatidylinositol and phosphatidylcholine head-group-containing lipids specifically. We further investigated the interaction of ZnONP with three physiologically relevant membrane conditions varying in composition and dipole potential. We found that ZnONP interaction leads to a photoinduced enhancement of the partial-to-complete phase separation depending upon the membrane composition and cholesterol content. Interestingly, while the lipid order of a partially-phase-separated membrane remained unchanged upon ZnONP crowding, a fully-phase-separated membrane showed an increase in the lipid order. Strikingly, ZnONP crowding induced a contrasting effect on the fluorescence anisotropy of the membrane upon binding to the two membrane conditions, in line with the measured diffusion coefficient. ZnONP seems to preferentially penetrate through the liquid disordered areas of the membrane and the boundaries of the phase-separated regions driven by the interplay between the electrostatics and phase boundary conditions, which are collectively dictated by the composition and ZnONP-induced lipid reorganization. The results may lead to a greater understanding of the interplay of membrane parameters and ZnONP interaction in driving passive penetration.


 Please wait while we load your content...
Please wait while we load your content...