Modelling the repair of carbon-centred protein radicals by the antioxidants glutathione and Trolox†
Abstract
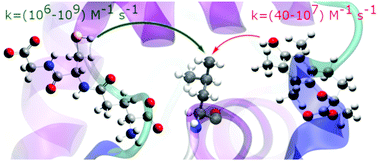
Repair is a procedure widely used in nature to heal a variety of biomolecules that have been damaged by oxidative stress. It is an important field of research with many unanswered questions. This article reports a computational kinetic and thermodynamic study of the chemical repair of radical-damaged leucine residues by the antioxidants glutathione (GSH) and Trolox via hydrogen-atom transfer under physiological conditions. The single-electron transfer mechanism is also considered for both antioxidants. Calculations are performed at the M06-2X-SMD/6-31++G(d,p) level of theory using the SMD solvation model to simulate polar and nonpolar environments. Calculated rate constants in water and pentyl ethanoate are reported. Our results show that GSH can repair carbon-centred protein radicals with rate constants in the diffusion limit, but Trolox repairs of this kind of radical are much slower and not likely to be physiologically relevant. Intrinsic barrier calculations confirm that the different reactivities of GSH and Trolox arise from the different strengths of their S–H and O–H bonds; antioxidants with thiol groups repair damaged proteins via hydrogen atom transfer reactions much faster than those with hydroxyl groups.


 Please wait while we load your content...
Please wait while we load your content...