Impact of individual flue gas components on mercury oxidation over a V2O5–MoO3/TiO2 catalyst
Abstract
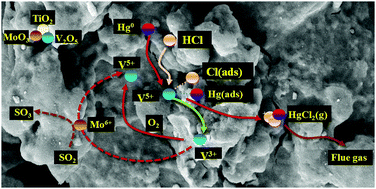
A titanium–vanadium–molybdenum (V2O5–MoO3/TiO2) SCR (selective catalytic reduction) catalyst has been confirmed to be effective for mercury oxidation in 6% O2/N2 atmosphere, but the influence of the actual flue gas components on mercury oxidation over this catalyst has not been reported. The individual flue gas components can significantly influence the mercury oxidation ability of the SCR catalyst. In this study, the effect of individual flue gas components on V2O5–MoO3/TiO2 for mercury oxidation was evaluated. The results suggested that the mercury oxidation efficiency of V2O5–MoO3/TiO2 was over 95% in the presence of 20 ppm HCl. The process of mercury oxidization by HCl over V2O5–MoO3/TiO2 followed the Langmuir–Hinshelwood mechanism. O2 was critical in this process, and we could continue catalytic recycling by re-oxidizing the reduced lattice oxygen. NH3 and H2O, especially H2O, suppressed the mercury oxidation ability of the catalyst. The mercury oxidation ability of V2O5–MoO3/TiO2 was almost lost in the presence of 8% H2O. Even after elimination of H2O, the mercury oxidation efficiency only recovered to 55%. This was due to the competition of NH3 or H2O with Hg0 for the same active sites on the surface of the catalyst. Unfortunately, H2O caused irreversible damage to the active sites. Additionally, the mercury oxidation efficiency was over 60% even with the existence of high SO2 content. This indicated that this catalyst can display some degree of sulfur resistance.


 Please wait while we load your content...
Please wait while we load your content...