The effect of calcination conditions on the crystal growth and battery performance of nanocrystalline Li(Ni1/3Co1/3Mn1/3)O2 as a cathode material for Li-ion batteries
Abstract
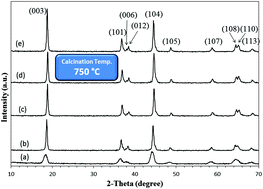
Nanocrystalline Li(Ni1/3Co1/3Mn1/3)O2 (NCM) was successfully synthesized through a solution combustion route to use as the cathode material in a Li-ion battery. The powder prepared by combustion was calcined at different temperatures of 750, 850 and 950 °C for 5–15 h. To evaluate the impact of the calcination time and temperature, the crystal growth kinetics of the obtained NCM material was investigated and the governing mechanism for the crystal growth was determined. The optimum material was discussed from both crystallographic and electrochemical standpoints.


 Please wait while we load your content...
Please wait while we load your content...