Copper(i)-based oxidation of polycyclic aromatic hydrocarbons and product elucidation using vacuum ultraviolet spectroscopy and theoretical spectral calculations†
Abstract
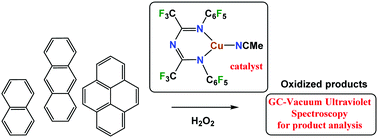
Copper(I) complexes supported by fluorinated 1,3,5-triazapentadienyl ligands have been used as catalysts for the oxidation of anthracene, naphthalene, and pyrene to the corresponding quinones, using H2O2 as an oxidant under mild conditions without an acid co-catalyst. Gas chromatography-vacuum ultraviolet spectroscopy (GC-VUV) combined with time-dependent density functional theory theoretical computations of absorption spectra was demonstrated as a new and useful tool-set for unknown determination in complex reaction mixtures, especially when standards are not available for spectral comparisons and product mixtures involve closely related isomers. The anthracene has been converted to 9,10-anthraquinone in quantitative yield using this copper catalyzed process. The oxidation of naphthalene afforded 1,4-naphthoquinone as the major product, and 1-naphthol and 2-naphthol as minor products. The pyrene oxidation resulted in 4,5-, 1,6-, and 1,8-pyrenequinones, among other products. The X-ray crystal structure of [N{(CF3)C(C6F5)N}2]CuNCCH3 is also reported.


 Please wait while we load your content...
Please wait while we load your content...