Computational investigations of intermolecular interactions between electron-accepting bromo- and iodo-pentafluorobenzene and electron-donating furan and thiophene
Abstract
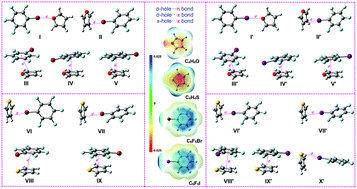
Bromo- and iodo-pentafluorobenzene (C6F5X, X = Br, I) exhibit intriguing σ- and π-hole characters, which are able to accept electrons, whereas furan (C4H4O) and thiophene (C4H4S) are able to donate electrons because of their electron-rich atoms and groups (i.e., the O atom and π-ring in C4H4O and the π-ring in C4H4S). Theoretical investigations of intermolecular interactions in these systems (i.e., C4H4O and C6F5Br, C4H4S and C6F5Br, C4H4O and C6F5I, and C4H4S and C6F5I) have been carried out at the M06-2X/aug-cc-pVDZ and wB97-XD/aug-cc-pVDZ levels with and without the counterpoise method, together with single-point calculations at the CCSD(T)/aug-cc-pVDZ level. For the system of C4H4O and C6F5X (X = Br, I), five different dimers, namely, I–V and I′–V′, which are connected by σ-hole⋯n, σ-hole⋯π and π-hole⋯π bonds, were found. Moreover, for the system of C4H4S and C6F5Br, four different dimers, namely, VI–IX, which are connected by σ-hole⋯π and π-hole⋯π bonds, were found. However, five dimers, namely, VI′–X′, were found for the system of C4H4S and C6F5I, which are connected by σ-hole⋯π, π-hole⋯π and σ-hole⋯n bonds. The driving forces for the formation of these dimers were revealed by EDA analyses. NCI, QTAIM and NBO analyses were employed to prove the existence of intermolecular interactions in these dimers and to elucidate their nature. The results presented here will be insightful for the study of other intermolecular systems that involve σ- and π-holes.


 Please wait while we load your content...
Please wait while we load your content...