Imidazole-containing cyanostilbene-based molecules with aggregation-induced emission characteristics: photophysical and electroluminescent properties†
Abstract
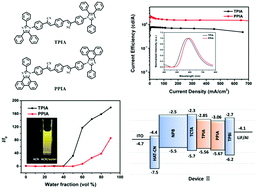
Organic luminogens with aggregation-induced emission (AIE) characteristics have recently attracted great research interest due to their excellent luminescence behavior in the aggregate state. Cyanostilbene is a well-known AIE-active moiety due to its unique luminescence mechanism and widespread use in the design of optical materials. In this work, we report two symmetrical imidazole-containing cyanostilbene derivatives with AIE activity, abbreviated as TPIA and PPIA, whose chemical structures only differ by a C–C bond. Both molecules are thermally stable and fabricated as electroluminescent (EL) devices. On one hand, the study of their photophysical properties confirms that the restricted intramolecular rotation (RIR) effect is still the dominant factor for the AIE effect in these two cyanostilbene-based molecules. On the other hand, the EL results reveal that a more conjugated molecular structure is beneficial for the EL properties. Moreover, the optimization of the device configuration with an electron-blocking layer significantly improves the EL performance. The orange-emissive PPIA-based device shows a high external quantum efficiency of 1.02% with a small efficiency roll-off.


 Please wait while we load your content...
Please wait while we load your content...