Synthesis of spiropyrrolidine oxindoles via Ag-catalyzed stereo- and regioselective 1,3-dipolar cycloaddition of indole-based azomethine ylides with chalcones†
Abstract
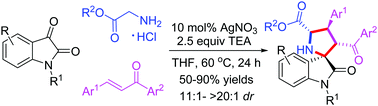
The synthesis of novel spiropyrrolidine oxindole derivatives was reported, using Ag-catalyzed [3+2] cycloaddition of azomethine ylides generated in situ from the condensation of substituted isatins and primary α-amino acid esters with chalcones. Products bearing four consecutive stereocenters, including spiroquaternary stereocenters fused in one ring structure, were smoothly acquired in moderate to high yields (50–95%) with good to excellent diastereoselectivities (11 : 1 → 20 : 1 dr). Furthermore, product 4a underwent reduction, oxidation, hydrolysis and amidization to give the corresponding alcohol, dihydropyrrole, pyrrole, acid and amide, respectively, in good yields. The synthesized compounds (>100 examples) were well characterized through different spectroscopic techniques, such as single crystal XRD, FTIR, NMR, and mass spectral analysis.


 Please wait while we load your content...
Please wait while we load your content...