Self-reversible mechanochromism and aggregation induced emission in neutral triarylmethanes and their application in water sensing†
Abstract
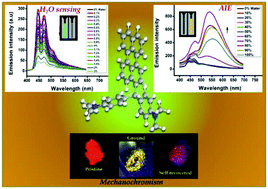
The photophysical properties and imaging/sensing applications of triarylmethane carbeniums are well recognised, whereas the neutral triarylmethane precursors are less investigated in this respect. Herein, we describe the synthesis of three triarylmethane derivatives, namely PhMBD, PyMBD and PrMBD, having a general structure R′R′′2CH, where R′ = phenanthrene, pyrene, and perylene, respectively and R′′ = N,N′-dimethylaniline. The photophysical studies of these molecules revealed the existence of intramolecular exciplexes even in solvents of moderate polarity. Supportive evidence for exciplex formation was obtained from TD-DFT calculations and solvent polarity/protonation-dependent absorption/emission profiles. Among the three, PrMBD exhibited high contrast and self-reversible mechanochromism with a hypsochromic shift of 74 nm on grinding. Moreover, it also showed time-dependent multicolour acidochromism in the solid state. PyMBD and PrMBD were aggregation induced emission (AIE) active and all three molecules responded to increasing viscosity of the medium with emission enhancement. The emission of these molecules was highly sensitive to solvent polarity and consequently responded to low levels of water in organic solvents with ‘turn off’ fluorescence.


 Please wait while we load your content...
Please wait while we load your content...