Neutral host–guest capsular associations by a homologous halophenyl-substituted organic tris-urea receptor series: solid and solution state studies†
Abstract
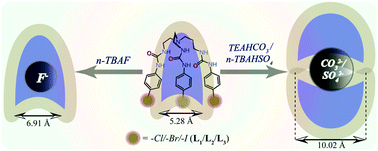
A set of three organic neutral tripodal scaffolds based on homologous p-halophenyl functionalization and decorated with hydrogen-bonding urea donor groups have been designed, synthesized and comprehensively studied as potential anion receptors in their neutral form. The three tris(2-aminoethyl)amine-based artificial receptors, tris([(4-chlorophenyl)amino]ethyl)-urea (L1), tris([(4-bromophenyl)amino]ethyl)-urea (L2) and tris([(4-iodophenyl)amino]ethyl)-urea (L3), effectively demonstrated the encapsulation of spherical, planar and tetrahedral anions in a systematic fashion through N–H⋯anion hydrogen-bonding interactions in the solid as well as in the solution state. Each tris-p-halophenyl urea isomer L1/L2/L3 showcases similar and constant proof of 1 : 1 receptor-fluoride encapsulation in complexes 1a/2a/3a, respectively, heavily corroborated by Hirshfeld surface analysis studies and detailed 1H-NMR solution state studies. On the other hand, each isomer has been structurally confirmed to self-assemble into a 2 : 1 dimeric molecular capsule in the presence of either planar carbonate or larger tetrahedral sulfate anions, irrespective of the size of the counter-cations in complexes 1b, 2b, 3b and 3c in the solid state, which is also supported by the solution state binding analysis. To the best of our knowledge, this report describes one of the first results on oxyanion encapsulation by any tren-based p-halophenyl urea receptor in the solid state.


 Please wait while we load your content...
Please wait while we load your content...