Photophysical studies of pyrenyl cyanostyrenes: effect of trifluoromethyl substitution on gelation†
Abstract
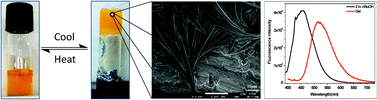
α-Cyanostyrenes bearing a planar pyrene unit and electron withdrawing trifluoromethyl units were designed and synthesized. The conformational restriction due to the presence of the cyano group on the double bond favors aggregation induced emission in aqueous media. The styrylpyrenes aggregate to form microstructures influenced by π–π stacking, cyano and CF3 substituent interactions. Importantly confluence of the pyrene ring and simple trifluoromethyl (CF3) unit allows the formation of a stable organogel with bathochromic shifts in emission. The formation of aggregates and the gel was substantiated using 1H NMR spectroscopy and scanning electron microscopy. The stability of the gels was assessed using rheology investigations and rationalized by single crystal X-ray data.


 Please wait while we load your content...
Please wait while we load your content...