Copper(i) tertiary phosphine xanthate complexes as single source precursors for copper sulfide and their application in the OER†
Abstract
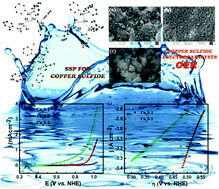
Three isomeric bis-(triphenylphosphine)copper(I) complexes comprising xanthate ligands derived from 2- (1), 3- (2), and 4-methanol pyridine (3) have been synthesized and characterized using microanalyses, IR, multinuclear NMR, UV-Vis and fluorescence spectroscopic methods as well as single crystal X-ray diffraction. The single crystal X-ray diffraction results indicated that the immediate geometry around Cu(I) in the complexes is distorted tetrahedral, which is satisfied by the two sulfur centers of the xanthate ligands and two phosphorus centers of the two PPh3 moieties. Additionally, thermogravimetric analysis (TGA) under an inert atmosphere was performed, and the results indicated that the complexes decompose to copper sulfide. The complexes were decomposed under an inert atmosphere to obtain copper sulfides, which were characterized by PXRD and SEM. Additionally, their electrocatalytic properties were investigated in the oxygen evolution reaction (OER). The OER results indicate that the copper sulfide (CuxS-3) obtained from 3 exhibited the best activity with an onset potential of 475 mV.


 Please wait while we load your content...
Please wait while we load your content...