Gold-catalyzed tandem reaction of 2-alkynylanilines followed by 1,6-conjugate addition to p-quinone methides: efficient access to unsymmetrical diarylindolylmethanes†
Abstract
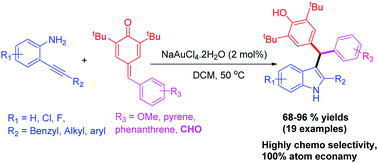
A simple, mild, efficient and chemoselective catalytic method for the straightforward synthesis of an interesting class of 2-aryl/alkyl-substituted-3-diaryl indolyl methanes in high yield is reported. This atom-efficient method proceeds via a gold-catalyzed one-pot sequential intramolecular hydroamination (C–N bond formation) of 2-alkynylanilines followed by a 1,6-conjugate addition to p-quinonemethides. The p-quinonemethides, which contain aldehyde functional groups, preferentially participate in 1,6-conjugate addition, while the aldehyde functional group remains unreactive.


 Please wait while we load your content...
Please wait while we load your content...