Facile one-pot synthesis of spirooxindole-pyrrolidine derivatives and their antimicrobial and acetylcholinesterase inhibitory activities†
Abstract
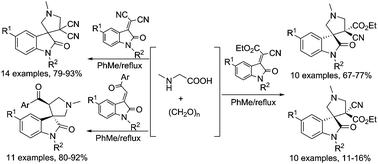
A library of novel spirooxindole-pyrrolidine derivatives were facilely synthesized via 1,3-dipolar cycloaddition of azomethine ylide generated from sarcosine and paraformaldehyde with various 3-methyleneoxindolines. The relative configuration of obtained spiro[indoline-3,3′-pyrrolidines] was successfully elucidated by determination of nine single-crystal structures. The antimicrobial activities and the inhibitory activity towards acetylcholinesterase of the synthesized spiro compounds were also preliminarily evaluated, in which the spiro[indoline-3,3′-pyrrolidine] 4d exhibited the most potent activity with an IC50 value of 69.07 ± 10.99 μM against acetylcholinesterase (AChE).


 Please wait while we load your content...
Please wait while we load your content...