Synthesis, structural, biological and in silico studies of new 5-arylidene-4-thiazolidinone derivatives as possible anticancer, antimicrobial and antitubercular agents†
Abstract
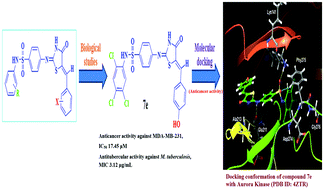
A new series of halogenated 4-thiazolidinone derivatives bearing the sulfonamide moiety was synthesized and characterized via FT-IR, 1H NMR, 13C NMR, HRMS and single crystal X-ray analysis. The newly synthesized target compounds were screened for their in vitro cytotoxicity on the HepG2 and MDA-MB-231 cell lines, and antimicrobial and antitubercular activity. The compounds showed promising anticancer activity towards the MDA-MB-231 cell line, and the trichloro derivatives with p-chloro substitution (6i) and p-hydroxy substitution (7e) exhibited excellent anticancer activity. Compounds 6b and 7c were observed to be moderate antimicrobial agents. The seven most potent anticancer agents were further studied for their antitubercular activity against an M. tuberculosis strain and it was found that compound 7e showed significant antitubercular activity. The potent candidates were also tested for hemolysis activity against human RBC cells and were found to be non-toxic. The mode of action for the observed anticancer activity was further supported by molecular docking studies of the potent compounds against the enzyme Aurora kinase (PDB ID: 4ZTR). Molecular dynamics (MD) simulations were further performed to study the stability of the ligand–protein complex.


 Please wait while we load your content...
Please wait while we load your content...