Iron molybdenum nitrilotriacetate and iminodiacetate – spectroscopy, structural characterization and CO2 adsorption†
Abstract
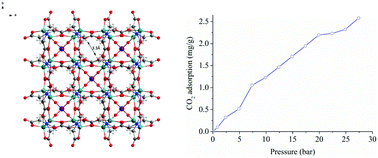
The tetranuclear iron molybdate Na6[(MoO2)2O2Fe2(nta)4]·16H2O (1) and decanuclear heterometallic polymer [(MoO4)2FeII4FeIII4(ida)8]n (2) were synthesized in modular manners (H3nta = nitrilotriacetic acid, H2ida = iminodiacetic acid). Isomorphous complexes of 1 were also obtained as Na6[(MoO2)2O2M2(nta)4]·16H2O (M = Al, 3; Cr, 4). Nta ligands in 1, 3 and 4 chelate to Mo atoms with two β-carboxy groups and one N atom, and the other β-carboxy group coordinates to adjacent heterometallic atoms(III) as a tetradentate ligand. The tetranuclear molybdenum(VI) nitrilotriacetates 1, 3 and 4 contain 2D water layers in their structures; the solution NMR spectra of 3 show no decomposition in solution. The decanuclear heterometallic polymer [(MoO4)2FeII4FeIII4(ida)8]n (2) forms an interesting cubic 3D microporous structure with alternating [MoO4] layers and [Fe(ida)] layers. There is a 3.3 Å diameter hole in the octanuclear structure of 2, which is formed by eight [Fe(ida)] units centered on MoO4. 2 is stable up to 250 °C and can adsorb a small amount of CO2.


 Please wait while we load your content...
Please wait while we load your content...