Perylenetetracarboxy-3,4:9,10-diimide derivatives with large two-photon absorption activity†
Abstract
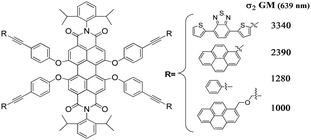
Three new perylenetetracarboxy-3,4:9,10-diimides, bearing 2,6-diisopropylphenyl groups at the imide positions and 4-(R-ethynyl)phenoxy moieties (R = 4,7-di(2-thienyl)benzo[c][1,2,5]thiadiazole (P2), pyrene (P3) or pyrene-CH2OCH2 (P4)) at the four bay positions, were prepared, along with the known related derivative (R = phenyl (P1)), and well characterized. They have large two-photon absorption (TPA) cross-sections (σ2), as determined by the Z-scan technique, the highest values being reached with P2 which bears a planar π-delocalized donor moiety. P3 is characterized by higher σ2 values than both P1, as expected for the higher π-conjugation of the donor pyrene moiety with respect to phenyl, and P4, due to the presence of the flexible and non-conjugated CH2OCH2 bridge between the pyrene and the ethynyl fragment in the latter compound. The molecular geometry of P1–P4 has been optimized by DFT modeling, showing that in P2 and P3 the bay substituents are stacked due to the π–π interactions of both pyrene and thiophene groups. The LUMO of P1–P4 lies at the same energy and is essentially delocalized on the perylene core whereas the HOMO and HOMO−1 of both P2 and P3 are degenerate and do not show contribution from the perylene core contrarily to that of P1 and P4. The HOMO–LUMO gap is therefore essentially influenced by the HOMO which reflects the electronic charge delocalization on the bay substituents, the lower gaps being observed for P2 and P3, which are characterized by the best TPA properties.


 Please wait while we load your content...
Please wait while we load your content...